Thermodynamics Miscellaneous
- One kg of an ideal gas (gas constant, R= 400 J/ kgK; specific heat at constant volume, cp = 1000 J/kgK) at 1 bar, and 300 K is contained in a sealed rigid cylinder. During an adiabatic process, 100 kJ of work is done on the system by a stirrer. The increase in entropy of the system is _______ J/K.
-
View Hint View Answer Discuss in Forum
Here,
m = 1 kg
R = 400 J/kgK
CV = 1000 J/kgK
P1 = 1 bar
T1 = 300 K
W = – 100 kJ = – 100 × 103 J
Q = 0
0 = – 100 × 103 + mCV (T2 – T1)
100 × 103= 1 × 1000 (T2 – 300)
T2 = 400 K
V1 = V2⇒ ln V2 = 0 V1 S2 - S1 = mCvln T2 T1 S2 - S1 = 1 × 1000 × ln 
400 
300
S2 – S1 = 287.68 J/KCorrect Option: A
Here,
m = 1 kg
R = 400 J/kgK
CV = 1000 J/kgK
P1 = 1 bar
T1 = 300 K
W = – 100 kJ = – 100 × 103 J
Q = 0
0 = – 100 × 103 + mCV (T2 – T1)
100 × 103= 1 × 1000 (T2 – 300)
T2 = 400 K
V1 = V2⇒ ln V2 = 0 V1 S2 - S1 = mCvln T2 T1 S2 - S1 = 1 × 1000 × ln 
400 
300
S2 – S1 = 287.68 J/K
- One kg of air (R = 287 J/kgK) undergoes an irreversible process between equilibrium state 1 (20°C, 0.9 m3) and equilibrium state 2 (20°C, 0.6 m3). The change in entropy s2 – s1 (in J/ kgK) is ______.
-
View Hint View Answer Discuss in Forum
– 116.36 J/kg– K
∆ S = mRln V2 V1 = (287) (1) ln 
0.6 
0.9
⇒ - 116.36 J/ kgKCorrect Option: A
– 116.36 J/kg– K
∆ S = mRln V2 V1 = (287) (1) ln 
0.6 
0.9
⇒ - 116.36 J/ kgK
- A closed system contains 10kg of saturated liquid ammonia at 10°C. Heat addition required to convert the entire liquid into saturated vapour at a constant pressure is 16.2 MJ. If the entropy of the saturated liquid is 0.88 kJ/kgK, the entropy (in kJ/kgK) of saturated vapour is____
-
View Hint View Answer Discuss in Forum
Tds = du + pdv (closed system)
283.15 × 10(S2 – S1) = 16.2 × 103∴ S2 = 0.88 + 16.2 × 103 283.15 × 10
= 6.6013 kJ/kgCorrect Option: A
Tds = du + pdv (closed system)
283.15 × 10(S2 – S1) = 16.2 × 103∴ S2 = 0.88 + 16.2 × 103 283.15 × 10
= 6.6013 kJ/kg
- An amount of 100 kW of heat is transferred through a wall in steady State. One side of the wall is maintained at 127°C and the other side at 27°C. The entropy generated (in W/K) due to the heat transfer through the wall is ______.
-
View Hint View Answer Discuss in Forum
∆S1 Q T1 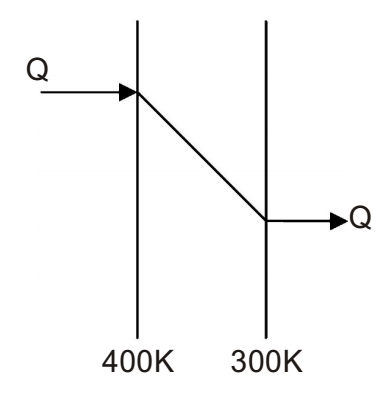
∆S2 Q T2
(∆S)generated = ∆S1 + ∆S2= Q - Q 400 300 
= - 83.33 W / KCorrect Option: A
∆S1 Q T1 
∆S2 Q T2
(∆S)generated = ∆S1 + ∆S2= Q - Q 400 300 
= - 83.33 W / K
- Which one of the following pairs of equations describes an irreversible heat engine?
-
View Hint View Answer Discuss in Forum
NA
Correct Option: A
NA

