Thermodynamics Miscellaneous
- If VN and α are the nozzle exit velocity and nozzle angle in an impulse turbine, the optimum blade velocity is given by
-
View Hint View Answer Discuss in Forum
NA
Correct Option: D
NA
- The following data pertain to a single stage impulse steam turbine: Nozzle angle = 20° Blade velocity = 200 m/s Relative steam velocity at entry = 350 m/s Blade inlet angle = 30° Blade exit angle = 25° If blade friction is neglected the work done per kg steam is
-
View Hint View Answer Discuss in Forum
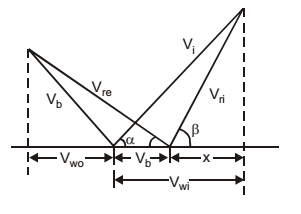
Given :α = 20°
β = 30°
x = 350 cos 30° = 303 m/s
∴ Vwi = 303 + Vb
= 303 + 200 = 503 m/scos 25° = 200 + Vwo = 0.9063 350
Vwo =118 m/s
Work done/kg of steam= (Vwi + Vwo) Vb kJ 1000 = (503 + 118) 200 = 124 kJ 1000
Correct Option: A

Given :α = 20°
β = 30°
x = 350 cos 30° = 303 m/s
∴ Vwi = 303 + Vb
= 303 + 200 = 503 m/scos 25° = 200 + Vwo = 0.9063 350
Vwo =118 m/s
Work done/kg of steam= (Vwi + Vwo) Vb kJ 1000 = (503 + 118) 200 = 124 kJ 1000
- A steam power plant has the boiler efficiency of 92%, turbine efficiency (mechanical) of 94%, generator efficiency of 95% and cycle efficiency of 44%. If 6% of the generated power is used to run the auxiliaries, the overall plant efficiency is
-
View Hint View Answer Discuss in Forum
η0 = ηb × ηm × ηs × ηc | 1 - ηa)
= 0.92 × 0.94 × 0.95 × 0.44r × (1 – 0.06)
η0 = 34%Correct Option: A
η0 = ηb × ηm × ηs × ηc | 1 - ηa)
= 0.92 × 0.94 × 0.95 × 0.44r × (1 – 0.06)
η0 = 34%
-
For a simple compressible system, v, s, p and T are specific volume, specific entropy, pressure and temperature, respectively. As per Maxwell’s relation, 
δV 
p is equal to δS
-
View Hint View Answer Discuss in Forum
dH = TdS + vdP
⇒ 
δT 
S = 
δV 
P δP δS So, ⇒ 
δV 
P = 
δT 
S δS δP
Correct Option: B
dH = TdS + vdP
⇒ 
δT 
S = 
δV 
P δP δS So, ⇒ 
δV 
P = 
δT 
S δS δP
- If one mole of H2 gas occupies a rigid container wi t h a capaci t y of 1000 l i t er s and t he temperature is raised from 27°C to 37°C, the change in pressure of the contained gas (round off to two decimal places), assuming ideal gas behavior, is _____ Pa. (R = 8.314 J/mol.K).
-
View Hint View Answer Discuss in Forum
P1 V = nRT1
P2 V = nRT1
Now, Changs in pressure,
P2 – P1 = nR (T2 - T1)
⇒ ∆P × 1 = 1 × 8.314(37 – 27)
⇒ ∆P = 83.14 PascalCorrect Option: A
P1 V = nRT1
P2 V = nRT1
Now, Changs in pressure,
P2 – P1 = nR (T2 - T1)
⇒ ∆P × 1 = 1 × 8.314(37 – 27)
⇒ ∆P = 83.14 Pascal

