Thermodynamics Miscellaneous
- An ideal gas of mass m and temperature T1 undergoes a reversible isothermal process from an initial pressure P1 to final pressure P2. The heat loss during the process is Q. The entropy change ∆S of the gas is
-
View Hint View Answer Discuss in Forum
For clausius theorem, ∮ δQ < 0 (for irreversible Heat engine). T
Correct Option: B
For clausius theorem, ∮ δQ < 0 (for irreversible Heat engine). T
Direction: In an experimental set-up, air flows between two stations P and Q adiabatically. The direction of flow depends on the pressure and temperature conditions maintained at P and Q. The conditions at station P are 150 kPa and 350 K. The temperature at station Q is 300 K. The following are the properties and relations pertaining to air:
Specific heat at constant pressure, cp = 1.005 kJ/kgK;
Specific heat at constant volume, cv = 0.718 kJ/kgK;
Characteristic gas constant, R = 0.287 kJ/kgK.
Enthalpy, h = cpT. .
Internal energy, u = cvT.
- If the pressure at station Q is 50 kPa, the change in entropy (sQ – sp) in kJ/kgK is
-
View Hint View Answer Discuss in Forum
∆s = δq for reversible process T 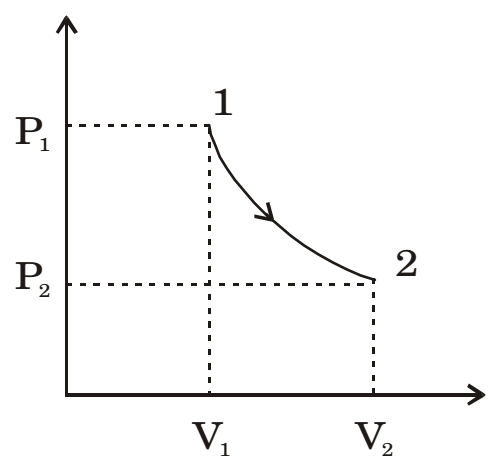
δQ = δW = P1V1ln V2 V1 ∆s = δq = mRln P1 T p2 ∆s = mR ln P1 P2
Correct Option: C
∆s = δq for reversible process T 
δQ = δW = P1V1ln V2 V1 ∆s = δq = mRln P1 T p2 ∆s = mR ln P1 P2
- If the air has to flow from station P to station Q, the maximum possible value of pressure in kPa at station Q is close to
-
View Hint View Answer Discuss in Forum
SQ - Sp = Cvln 
T2 
+ Rln 
V2 
T1 V1
From perfect gas law,V2 = P1 T2 V1 P2 T1 = 150 × 300 = 2.57 300 × 350
∴ SQ – Sp = – 0.1107 + 0.287 ln 2.57 = 0.16 kJ/kgK
Correct Option: B
SQ - Sp = Cvln 
T2 
+ Rln 
V2 
T1 V1
From perfect gas law,V2 = P1 T2 V1 P2 T1 = 150 × 300 = 2.57 300 × 350
∴ SQ – Sp = – 0.1107 + 0.287 ln 2.57 = 0.16 kJ/kgK
- One kilogram of water at room temperature is brought into contact with a high temperature thermal reservoir. The entropy change of the universe is
-
View Hint View Answer Discuss in Forum
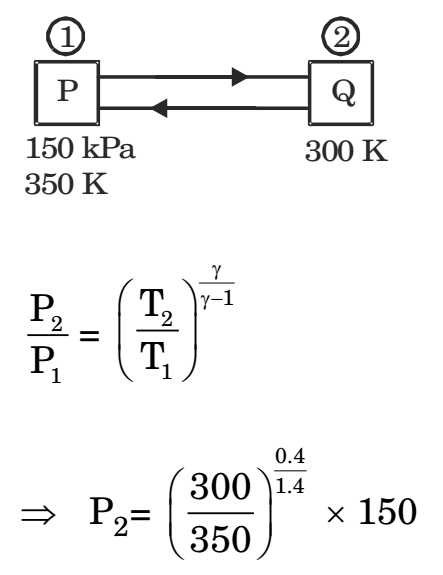
P2 = 87.4 kPa
P2 = 87kPaCorrect Option: D

P2 = 87.4 kPa
P2 = 87kPa
- A rope-brake dynamometer attached to the crank shaft of an I.C. engine measures a brake power of 10 kW when the speed of rotation of the shaft is 400 rad/s. The shaft torque (in Nm) sensed by the dynamometer is _____.
-
View Hint View Answer Discuss in Forum
P = Tω
∴ T = P = 10000 = 25 N-m ω 400
Correct Option: A
P = Tω
∴ T = P = 10000 = 25 N-m ω 400

