Thermodynamics Miscellaneous
- A small steam whistle (perfectly insulated and doing no shaft work) causes a drop of 0.8 kJ/kg in enthalpy of steam from entry to exit. If the kinetic energy of the steam at entry is negligible, the velocity of the steam at exit is
-
View Hint View Answer Discuss in Forum
v²1 + h1 = v²2 2 2
Heres v1 = 0, thereforeh1 = v²2 + h2 2
or v2 = √2(h1 - h2) = √2 × 0.8 × 1000 = 40 m/sec.Correct Option: B
v²1 + h1 = v²2 2 2
Heres v1 = 0, thereforeh1 = v²2 + h2 2
or v2 = √2(h1 - h2) = √2 × 0.8 × 1000 = 40 m/sec.
- A steam turbine receives steam steadily at 10 bar with a enthalpy of 3000 kJ/kg and discharges at 1 bar with an enthalpy of 2700 kJ/kg. The work output is 250 kJ/kg. The changes in kinetic and potential energies are negligible. The heat transfer from the turbine casing to the surroundings is equal to
-
View Hint View Answer Discuss in Forum
∆h = ∆Q – ∆W
∴ ∆Q = (– 300 + 250) kJ/kg = – 50 kJ/kgCorrect Option: B

∆h = ∆Q – ∆W
∴ ∆Q = (– 300 + 250) kJ/kg = – 50 kJ/kg
- An engine working on air standard Otto cycle is supplied with air at 0.1 MPa and 35°C. The compression ratio is 8. The heat supplied is 500 kJ/kg. Property data for air: cp = 1.005 kJ/ kgK, cv = 0.718 kJ/kgK, R = 0.287 kJ/kgK. The maximum temperature (in K) of the cycle is ________(correct to one decimal place).
-
View Hint View Answer Discuss in Forum
P1 = 0.1 MPa
T1 = 35°C = 35 + 273 = 308 K
Heat Supplied Qs = Cv (T3 – T2)
Cv (T3 – T2) = 500 kJ/kg ..............(i)
Given, 
V2 
γ - 1 = T1 V1 T2 ⇒ 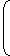
1 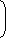
1.4 - 1 = 308 = 707.60K 8 T2
⇒ T2 = 707.60 K
After putting the value of T2 is equation(i):-
0.718 (T3 – 707.6) = 500
⇒ T3 = 1403.98 KCorrect Option: C
P1 = 0.1 MPa
T1 = 35°C = 35 + 273 = 308 K
Heat Supplied Qs = Cv (T3 – T2)
Cv (T3 – T2) = 500 kJ/kg ..............(i)
Given, 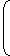
V2 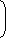
γ - 1 = T1 V1 T2 ⇒ 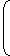
1 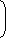
1.4 - 1 = 308 = 707.60K 8 T2
⇒ T2 = 707.60 K
After putting the value of T2 is equation(i):-
0.718 (T3 – 707.6) = 500
⇒ T3 = 1403.98 K
- An air-standard Diesel cycle consists of the following processes: 1-2: Air is compressed isentropically. 2-3: Heat is added at constant pressure. 3-4: Air expands isentropically to the original volume. 4-1: Heat is rejected at constant volume. If γ and T denote the specific heat ratio and temperature, respectively, the efficiency of the cycle is
-
View Hint View Answer Discuss in Forum
Heat applied,Qs = cp (T3 – T2)
Heat rejected, Qr = cV (T4 – T1)
η = 1 - Qr = 1 - 1 (T4 - T1) Qs γ (T3 - T2) Correct Option: B
Heat applied,Qs = cp (T3 – T2)
Heat rejected, Qr = cV (T4 – T1)
η = 1 - Qr = 1 - 1 (T4 - T1) Qs γ (T3 - T2)
- For the same values of peak pressure, peak temperature and heat rejection, the correct order of efficiencies for Otto, Duel and Diesel cycles is
-
View Hint View Answer Discuss in Forum
For same values of peak pressure and temperature. Diesel cycle is most efficient and Otto cycle is least. Efficiency of dual cycle lies in between.ηDiesel > ηDual > ηOtto
Correct Option: B
For same values of peak pressure and temperature. Diesel cycle is most efficient and Otto cycle is least. Efficiency of dual cycle lies in between.ηDiesel > ηDual > ηOtto

