Thermodynamics Miscellaneous
- Propane (C3H8) is burned in an oxygen atmosphere with 10% deficit oxygen with respect to the stoichiometric requirement. Assuming no hydrocarbons in the products, the volume percentage of CO in the products is___.
-
View Hint View Answer Discuss in Forum
C3 H8 + xO2 → aCO2 + bH2 O
Carbon balance: a = 3
Hydrogen balance: 2b = 8 → b = 4
Oxygen balance:
2x = 2a + b→ x = a + b = 3 + 4 = 5 2 2
For chemical correct or stoichiometric burning, no of moles of O2 required are = 5.
As it is burnt with 10% deficient oxygen, it will generate CO.
The new equation is
C3H8 + 0.9 × 5O2 → CO2 + bCO+ cH2O
Carbon balance: a + b = 3
Hydrogen balance: 2c = 8 → c = 4
Oxygen balance:
2a + b + c = 0.9 × 5 × 2 = 9
2a + b + c = 9
⇒ 2a + b + c ⇒ 2a + b = 5
a + b = 3
By solving these two equations a = 2 and b = 1
In the exhaust products the no, of moles of CO are 1.% by volume of CO in exhaust,= b × 100 = 1 × 100 a + b + c 2 + 1 + 4 = 1 × 100 = 14.29% 7
Correct Option: A
C3 H8 + xO2 → aCO2 + bH2 O
Carbon balance: a = 3
Hydrogen balance: 2b = 8 → b = 4
Oxygen balance:
2x = 2a + b→ x = a + b = 3 + 4 = 5 2 2
For chemical correct or stoichiometric burning, no of moles of O2 required are = 5.
As it is burnt with 10% deficient oxygen, it will generate CO.
The new equation is
C3H8 + 0.9 × 5O2 → CO2 + bCO+ cH2O
Carbon balance: a + b = 3
Hydrogen balance: 2c = 8 → c = 4
Oxygen balance:
2a + b + c = 0.9 × 5 × 2 = 9
2a + b + c = 9
⇒ 2a + b + c ⇒ 2a + b = 5
a + b = 3
By solving these two equations a = 2 and b = 1
In the exhaust products the no, of moles of CO are 1.% by volume of CO in exhaust,= b × 100 = 1 × 100 a + b + c 2 + 1 + 4 = 1 × 100 = 14.29% 7
- At the time of starting, idling and low speed operation, the carburettor supplies a mixture which can be termed as
-
View Hint View Answer Discuss in Forum
Very rich mixture is provided during starting, idling and peak power to ensure burning due to low supply of air, low temperature etc.
Correct Option: D
Very rich mixture is provided during starting, idling and peak power to ensure burning due to low supply of air, low temperature etc.
- A diesel engine is usually more efficient than a spark ignition engine because
-
View Hint View Answer Discuss in Forum
NA
Correct Option: C
NA
Direction: In a simple Brayton cycle, the pressure ratio is 8 and temperature at the entrance of compressor and turbine are 300 K and 1400 K, respectively. Both compressor and gas turbine have isentropic efficiencies equal to 80%. For the gas, assume a constant value of cp (specific heat at constant pressure) equal to 1 kJ/kgK and ratio of specific heats as 1.4. Neglect changes in kinetic and potential energies.
- In a 3-stage air compressor, the inlet pressure is p1, discharge pressure is p4 and the intermediate pressures are p2 and p3 (p2 < p3). The total pressure ratio of the compressor is 10 and the pressure ratios of the stages are equal. If p1 = 100 kPa, the value of the pressure p3 (in kPa) is _______.
-
View Hint View Answer Discuss in Forum
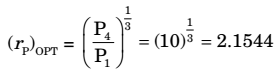
P2 = P3 = P4 = 2.1544 P1 P2 P3
P2 = 2.1544 × 100 = 215.44P3 = 2.1544 P2
P3 = 2.1544 × 215.44 = 464.15 kPaCorrect Option: A
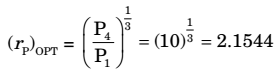
P2 = P3 = P4 = 2.1544 P1 P2 P3
P2 = 2.1544 × 100 = 215.44P3 = 2.1544 P2
P3 = 2.1544 × 215.44 = 464.15 kPa
- The thermal efficiency of the cycle in percentage (%) is
-
View Hint View Answer Discuss in Forum
T4 = T3 = 772.9K 8.2857 ηT = T3 - T4' ⇒ 0.8 = 1400 - T4' T3 - T4 1400 - 772.9 ηcycle = (WT)actual - (WC)actual Qs = (T3 - T4') - (T2' - T1) T3 - T2' ηcycle= (1400 - 898.32) - (604.3 - 300) 1400 - 604.3
= .248 or 24.8%Correct Option: A
T4 = T3 = 772.9K 8.2857 ηT = T3 - T4' ⇒ 0.8 = 1400 - T4' T3 - T4 1400 - 772.9 ηcycle = (WT)actual - (WC)actual Qs = (T3 - T4') - (T2' - T1) T3 - T2' ηcycle= (1400 - 898.32) - (604.3 - 300) 1400 - 604.3
= .248 or 24.8%

