-
One kg of an ideal gas (gas constant, R= 400 J/ kgK; specific heat at constant volume, cp = 1000 J/kgK) at 1 bar, and 300 K is contained in a sealed rigid cylinder. During an adiabatic process, 100 kJ of work is done on the system by a stirrer. The increase in entropy of the system is _______ J/K.
-
- 287.68 J/K
- 187.68 J/K
- 387.68 J/K
- 277.68 J/K
Correct Option: A
Here,
m = 1 kg
R = 400 J/kgK
CV = 1000 J/kgK
P1 = 1 bar
T1 = 300 K
W = – 100 kJ = – 100 × 103 J
Q = 0
0 = – 100 × 103 + mCV (T2 – T1)
100 × 103= 1 × 1000 (T2 – 300)
T2 = 400 K
V1 = V2
| ⇒ ln | = 0 | V1 |
| S2 - S1 = mCvln | T1 |
| S2 - S1 = 1 × 1000 × ln | 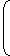 | 400 | 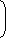 | |
| 300 |
S2 – S1 = 287.68 J/K

