Thermodynamics Miscellaneous
- An ideal Brayton cycle, operating between the pressure limits of 1 bar and 6 bar, has minimum and maximum temperatures of 300 K and 1500 K. The ratio of specific heats of the working fluid is 1.4. The approximate final temperature in Kelvin at the end of the compression and expansion processes are respectively
-
View Hint View Answer Discuss in Forum
Ideal Brayton cycle,
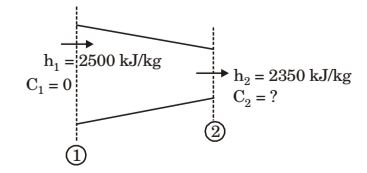
At the end of compression,Temperature, T2 = T1 
P3 
γ - 1 /γ T4
= 300 × 60.4/1.4 = 500 K
At the end of expansion;Temperature, T4 = T3 
P3 
γ-1/γ P4 = 1500 60.4/1.4
= 900KCorrect Option: A
Ideal Brayton cycle,

At the end of compression,Temperature, T2 = T1 
P3 
γ - 1 /γ T4
= 300 × 60.4/1.4 = 500 K
At the end of expansion;Temperature, T4 = T3 
P3 
γ-1/γ P4 = 1500 60.4/1.4
= 900K
- The values of enthalpy of steam at the inlet and outlet of a steam turbine in a Rankine cycle are 2800 kJ/kg and 1800 kJ/kg respectively. Neglecting pump work, the specific steam consumption in kg/kW hour is
-
View Hint View Answer Discuss in Forum
41. Work done by the turbine
W = 2800 – 1800 = 1000 kJ/kg
= 1000 kW-s/kgSpecific fuel consumption = 1 × 3600 1000
= 3.6 kg/kW – hrCorrect Option: A
41. Work done by the turbine
W = 2800 – 1800 = 1000 kJ/kg
= 1000 kW-s/kgSpecific fuel consumption = 1 × 3600 1000
= 3.6 kg/kW – hr
Direction: The inlet and the outlet conditions of steam for an adiabatic steam turbine are as indicated in the notations are as usually followed. 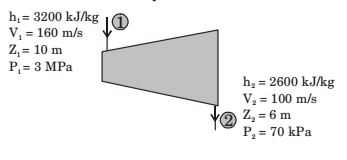
- Assume the above turbine to be part of a simple Rankine cycle. The density of water at the inlet to the pump is 1000 kg/m3. Ignoring kinetic and potential energy effects, the specific work (in kJ/kg) supplied to the pump is
-
View Hint View Answer Discuss in Forum
Pump work
hinlet + q = houtlet + Wpump
here, q = 0
Wpump = hinlet – houtlet
∴ W = ∆hpump
Reversible adiabatic
dq = dh– Vdp
∴ dh = Vdp
∴ dhpump =V(p2 – p1) = 1/ρ (3000–70) kPa = 2.930kPaCorrect Option: C
Pump work
hinlet + q = houtlet + Wpump
here, q = 0
Wpump = hinlet – houtlet
∴ W = ∆hpump
Reversible adiabatic
dq = dh– Vdp
∴ dh = Vdp
∴ dhpump =V(p2 – p1) = 1/ρ (3000–70) kPa = 2.930kPa
- If mass flow rate of steam through the turbine is 20 kg/s, the power output of the turbine (in MW) is
-
View Hint View Answer Discuss in Forum
SFEE h1 + z1g + c²1 + Q = h2 + z2g + c²2 + W 2 2
For adiabatic process, Q = 0∴ h2 + z1g + c²1 = h2 + z2g + c²2 + W 2 2 Or 3200 × 10³ + 10 × 9.81 + 160 × 160 2 = 2600 × 10³ + 6 × 9.81 + 100 × 100 + W 2 ⇒ W = 600 × 10³ + 39.24 + 2600 - 1000 + W 2
= 607839.24 × 20 J/s = 12.156 MW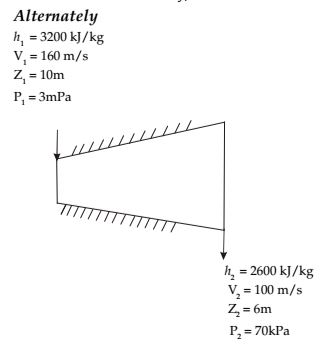
Applying S.F.E.Ew1 
h1 + V²1 + gz1 
+ dQ w2 
h2 + V²2 + gz2 
+ δw 2 dτ 2 dτ
Here, w1 = mass flow ratedQ = 0 for insulated turbine dτ ∴ 20 
3200 + 160² × 9.81 × 10 
2 × 10³ = 20 
2600 + 160² + 9.81 × 6 
+ dw 2 × 10³ 10³ dτ Or dw = 20 
(3200 - 2600) + 1 
160² - 100² 
+ 9.81(10 - 6) 
dτ 10³ 2 2 10³
= 20 × {600 + 7.8 + 0.03924}
= 20 × 607.83924 kW
= 12156.7848 kW ≃ 12.157MWCorrect Option: A
SFEE h1 + z1g + c²1 + Q = h2 + z2g + c²2 + W 2 2
For adiabatic process, Q = 0∴ h2 + z1g + c²1 = h2 + z2g + c²2 + W 2 2 Or 3200 × 10³ + 10 × 9.81 + 160 × 160 2 = 2600 × 10³ + 6 × 9.81 + 100 × 100 + W 2 ⇒ W = 600 × 10³ + 39.24 + 2600 - 1000 + W 2
= 607839.24 × 20 J/s = 12.156 MW
Applying S.F.E.Ew1 
h1 + V²1 + gz1 
+ dQ w2 
h2 + V²2 + gz2 
+ δw 2 dτ 2 dτ
Here, w1 = mass flow ratedQ = 0 for insulated turbine dτ ∴ 20 
3200 + 160² × 9.81 × 10 
2 × 10³ = 20 
2600 + 160² + 9.81 × 6 
+ dw 2 × 10³ 10³ dτ Or dw = 20 
(3200 - 2600) + 1 
160² - 100² 
+ 9.81(10 - 6) 
dτ 10³ 2 2 10³
= 20 × {600 + 7.8 + 0.03924}
= 20 × 607.83924 kW
= 12156.7848 kW ≃ 12.157MW
- A compressor undergoes a reversible, steady flow process. The gas at inlet and outlet of the compressor is designated as state 1 and state 2 respectively. Potential and kinetic energy changes are to be ignored. The following notations are used: v = specific volume and P = pressure of the gas. The specific work required to be supplied to the compressor for this gas compression process is
-
View Hint View Answer Discuss in Forum
h = u + Pv
⇒ dh =du + Pdv + vdP
But dQ =du + Pdv
∴ du = dQ –Pdv
∴ dh = dQ – Pdv + Pdv + vdP
⇒ dh = dQ + vdP
⇒ –vdP = d(P.E) + d(K.E) + dW
Since K.E and P.E are constant
∴ dW = ∫²1vdPCorrect Option: B
h = u + Pv
⇒ dh =du + Pdv + vdP
But dQ =du + Pdv
∴ du = dQ –Pdv
∴ dh = dQ – Pdv + Pdv + vdP
⇒ dh = dQ + vdP
⇒ –vdP = d(P.E) + d(K.E) + dW
Since K.E and P.E are constant
∴ dW = ∫²1vdP

