Thermodynamics Miscellaneous
- When a system executes an irreversible cycle
-
View Hint View Answer Discuss in Forum
∮ δQ < 0 clausius inequality T
Correct Option: A
∮ δQ < 0 clausius inequality T
- Any thermodynamic cycle operating between two temperature limits is reversible if the product of the efficiency when operating as a heat engine and the COP when operating as a refrigerator is equal to l.
-
View Hint View Answer Discuss in Forum
False
ηHE × COPHP = 1
Where , HP = Heat pumpCorrect Option: C
False
ηHE × COPHP = 1
Where , HP = Heat pump
- Figure below shows a reversible heat engine ER having heat interactions with three constant temperature systems. Calculate the thermal efficiency of the heat engine
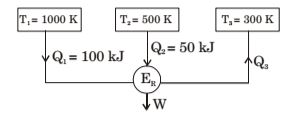
-
View Hint View Answer Discuss in Forum
Q1 + Q2 - Q3 = W .... (i)
Q1 + Q2 - Q3 = 0 ...... (ii) 1000 500 300
From equation (ii) , we haveQ1 + Q2 - Q3 = 0 1000 500 300
⇒ Q3 = 60 kJ
W ⇒ 100 + 50 - 60 = 90 kJ
η = W = 90 = 60% Q1 + Q2 150
Correct Option: A
Q1 + Q2 - Q3 = W .... (i)
Q1 + Q2 - Q3 = 0 ...... (ii) 1000 500 300
From equation (ii) , we haveQ1 + Q2 - Q3 = 0 1000 500 300
⇒ Q3 = 60 kJ
W ⇒ 100 + 50 - 60 = 90 kJ
η = W = 90 = 60% Q1 + Q2 150
- A solar energy based heat engine which receives 80 kJ of heat at 100°C and rejects 70 kJ of heat to the ambient at 30°C is to be designed. The thermal efficiency of the heat engine is
-
View Hint View Answer Discuss in Forum
ηth = ( 80 - 70 ) × 100 = 1 × 100 = 12.5% 80 8 Correct Option: C
ηth = ( 80 - 70 ) × 100 = 1 × 100 = 12.5% 80 8
- For two cycles coupled in series, the topping cycle has an efficiency of 30% and the bottoming cycle has an efficiency of 20%. The overall combined cycle efficiency is
-
View Hint View Answer Discuss in Forum
η0 = η1 + η2 - η1η2
η0 = 0.3 + 0.2 – (0.3) (0.2) = 0.44 = 44%Correct Option: B
η0 = η1 + η2 - η1η2
η0 = 0.3 + 0.2 – (0.3) (0.2) = 0.44 = 44%

