Thermodynamics Miscellaneous
- Nitrogen gas (molecular weight 28) is enclosed in a cylinder by a piston, at the initial condition of 2 bar, 298 K and 1 m3. In a particular process, the gas slowly expands under isothermal condition, until the volume becomes 2 m3. Heat exchange occurs with the atmosphere at 298 K during this process.
The work interaction for the nitrogen gas is
-
View Hint View Answer Discuss in Forum
Work interaction , 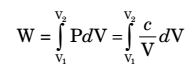
W = cln 
V2 
V1 W = P1V1ln 
V2 
V1
W = 2 × 105 × ln(2)
W = 2 × 105 × 0.693
W = 1.386 × 105 J = 138.6 kJCorrect Option: B
Work interaction , 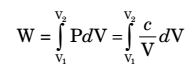
W = cln 
V2 
V1 W = P1V1ln 
V2 
V1
W = 2 × 105 × ln(2)
W = 2 × 105 × 0.693
W = 1.386 × 105 J = 138.6 kJ
- A 2 kW, 40 litres water heater is switched on for 20 minutes. The heat capacity cp for water is 4.2 kJ/kgK. Assuming all the electrical energy has gone into heating the water, increase of the water temperature in degree centigrade is
-
View Hint View Answer Discuss in Forum
Energy given by water = Energy taken by heater P.t = mcp ∆T = 2 × 20 × 60 = 40 × 4.2 × ∆t ∆t = 14.3 °C
Correct Option: C
Energy given by water = Energy taken by heater P.t = mcp ∆T = 2 × 20 × 60 = 40 × 4.2 × ∆t ∆t = 14.3 °C
- A vertical cylinder with a freely floating piston contains 0.1 kg air at 1.2 bar and a small electrical resistor. The resistor of is wired to an external 12 volt battery. When a current of 1.5 amps is passed through the resistor for 90 secs, the piston sweeps a volume of 0.01 m3. Assume
(i) piston and the cylinder are insulated and (ii) air behaves as an ideal gas with cv = 700 J/kgK,
Find the rise in temperature of air.
-
View Hint View Answer Discuss in Forum
m = 0.1 kg , P = 1.2 bar , Voltage E = 12 volt I = 1.5 A , t = 90 sec ∆v = 0.01/m3 , Cv = 700 J/kg–K
Insulated condition δq = 0
δq = δw + ∆U
0 = mCv∆T + δwair - δwe∆T = δwair - δwe mCv ∆T = 1.2 × 105 × 0.01 - 12 × 1.5 × 90 0.1 × 700 Correct Option: E
m = 0.1 kg , P = 1.2 bar , Voltage E = 12 volt I = 1.5 A , t = 90 sec ∆v = 0.01/m3 , Cv = 700 J/kg–K
Insulated condition δq = 0
δq = δw + ∆U
0 = mCv∆T + δwair - δwe∆T = δwair - δwe mCv ∆T = 1.2 × 105 × 0.01 - 12 × 1.5 × 90 0.1 × 700
- A steel ball of mass 1 kg of specific heat 0.4 kJ/ kgK is at a temperature of 60°C. It is dropped into 1 kg water at 20°C. The final steady state temperature of water is
-
View Hint View Answer Discuss in Forum
N/A
Correct Option: E
N/A
- The first law of thermodynamics takes the form W = –∆H when applied to
-
View Hint View Answer Discuss in Forum
Steady flow equation is given be,
H1 + q = H2 + W
Adiabatic q = 0
H1 – H2 = W
W = -∆HCorrect Option: B
Steady flow equation is given be,
H1 + q = H2 + W
Adiabatic q = 0
H1 – H2 = W
W = -∆H

