Thermodynamics Miscellaneous
Direction: The following table of properties was printed out for saturated liquid and saturated vapour of ammonia. The title for only the first two columns are available. All that we know that the other columns (columns 3 to 8) contain data on specific properties, namely, internal energy (kJ/ kg), enthalpy (kJ/kg) and entropy (kJ/kgK). 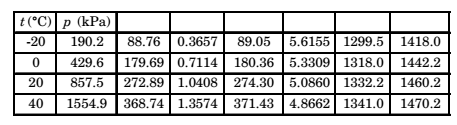
- when saturated liquid at 40°C is throttled to – 20°C, the quality at exit will be
-
View Hint View Answer Discuss in Forum
NA
Correct Option: B
NA
- The specific enthalpy data are in columns
-
View Hint View Answer Discuss in Forum
NA
Correct Option: D
NA
Direction: In two air standard cycles - one operating in the Otto and the other on the Brayton cycle - air is isentropically compressed from 300 to 450 K. Heat is added to raise the temperature to 600 K in the Otto cycle and to 550 K in the Brayton cycle.
- If W0 and WB are work outputs per unit mass, then
-
View Hint View Answer Discuss in Forum
W0 = η0CV × 150 = 35.54 kJ/kg
WB = η0CP × 100 = 33.165 kJ/kgCorrect Option: A
W0 = η0CV × 150 = 35.54 kJ/kg
WB = η0CP × 100 = 33.165 kJ/kg
- In η0 and ηb are the efficiencies of the Otto and Brayton cycles, then
-
View Hint View Answer Discuss in Forum
η0 = η0 = 1 - T1 = 1 - 300 = 1.3 T2 450 = 150 = 0.333 450
[ ηB = η0 = 33.3% ]Correct Option: B
η0 = η0 = 1 - T1 = 1 - 300 = 1.3 T2 450 = 150 = 0.333 450
[ ηB = η0 = 33.3% ]
Direction: Consider a steam power plant using a reheat cycle as shown. Steam leaves the boiler and enters the turbine at 4 MPa, 350°C (h3 = 3095 kJ/kg). After expansion in the turbine to 400 kPa (h4 = 2609 kJ/kg), the steam is reheated to 350°C (h5 = 3170 kJ/kg), and then expanded in a low pressure turbine to 10 kPa (h6 = 2165 kJ/kg) the specific volume of liquid handled by the pump can be assumed to be 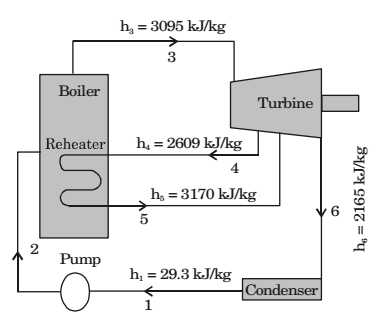
- The enthalpy at the pump discharge (h2) is
-
View Hint View Answer Discuss in Forum
Enthalpy at the pump discharge will be greater than 29.3 kJ/kg
Hence from given choice clearly we can say
h2 = 33.3 kJ/kg.Correct Option: D
Enthalpy at the pump discharge will be greater than 29.3 kJ/kg
Hence from given choice clearly we can say
h2 = 33.3 kJ/kg.

