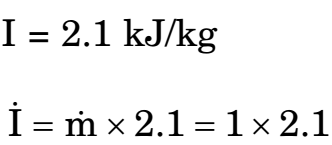Thermodynamics Miscellaneous
- Constant pressure lines in the superheated region of the Mollier diagram will have
-
View Hint View Answer Discuss in Forum
Mollier diagram is h-s diagram
Tds = dh – vdP
dP = 0
Tds = dhdh = T ds
∵ T > 0dh > 0 ds
slope > 0Correct Option: A
Mollier diagram is h-s diagram
Tds = dh – vdP
dP = 0
Tds = dhdh = T ds
∵ T > 0dh > 0 ds
slope > 0
- The relationship (δT / δP)h = 0 holds good for
-
View Hint View Answer Discuss in Forum
is Joule-Thompson coefficient (μ)= 
δT 
h δP
= 0 for an ideal gas, at any state for any gas at its inversion point. Inversion curve is the locus of all the point at which μ is zero.= 
δT 
h δP
Correct Option: E
is Joule-Thompson coefficient (μ)= 
δT 
h δP
= 0 for an ideal gas, at any state for any gas at its inversion point. Inversion curve is the locus of all the point at which μ is zero.= 
δT 
h δP
- A tank of volume 0.05 m3 contains a mixture of saturated water and saturated steam at 200°C. The mass of the liquid present is 8 kg. The entropy (in kJ/kgK) of the mixture is ________ (correct of two decimal places) Property data for saturated steam and water are: At 200ºC [Psat = 1.5538 MPa] vf = 0.001157 m3/kg, vg = 0.12736 m3/kg sfg = 4.1014 kJ/kgK, sf = 2.3309 kJ/kgK
-
View Hint View Answer Discuss in Forum
Total volume of tank (V) = 0.05 m3
Means of liquid (m²) = 8 kg
VV = mV VV
VV = Vg @ 200°C
= 0.12736 m3 /kg
VV = mV LL
VL = Vf @ 200°C
= 0.001157 m3 /kg
VL = 8 × 0.001157
= 9.256 × 10-3 m3
0.05 = 9.256 × 10-3 + VV
VV = 0.0474 m3
0.04074 = mV × 0.12736
mV = 0.3198 kgx = 0.3198 0.3198 + 8
⇒x = 0.0384
Specific entropy of mixture(s)
s = sf + xsfg
s = 2.3309 + 0.0384 × 4.1014
s = 2.4884 kJ/kg-KCorrect Option: A
Total volume of tank (V) = 0.05 m3
Means of liquid (m²) = 8 kg
VV = mV VV
VV = Vg @ 200°C
= 0.12736 m3 /kg
VV = mV LL
VL = Vf @ 200°C
= 0.001157 m3 /kg
VL = 8 × 0.001157
= 9.256 × 10-3 m3
0.05 = 9.256 × 10-3 + VV
VV = 0.0474 m3
0.04074 = mV × 0.12736
mV = 0.3198 kgx = 0.3198 0.3198 + 8
⇒x = 0.0384
Specific entropy of mixture(s)
s = sf + xsfg
s = 2.3309 + 0.0384 × 4.1014
s = 2.4884 kJ/kg-K
- Which one of the following statements is correct for a superheated vapour?
-
View Hint View Answer Discuss in Forum

Correct Option: A

- Water flowing at the rate of 1 kg/s through a system is heated using an electric heater such that the specific enthalpy of the water increases by 2.50 kJ/kg and the specific entropy increases by 0.007 kJ/kg.K. The power input t o t he electric heater is 2.50 kW. There is no other work or heat interaction between the system and the surroundings. Assuming an ambient temperature of 300 K, the irreversibility rate of the system is ____ kW (round off to two decimal places)
-
View Hint View Answer Discuss in Forum
Irreversibility of system
= T0 .(∆ S)syst = 300[0.007]
I = 2.1 kJ/kg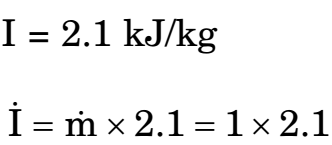
Correct Option: A
Irreversibility of system
= T0 .(∆ S)syst = 300[0.007]
I = 2.1 kJ/kg