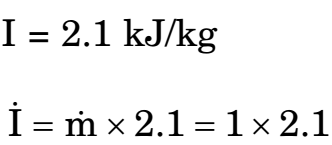-
Water flowing at the rate of 1 kg/s through a system is heated using an electric heater such that the specific enthalpy of the water increases by 2.50 kJ/kg and the specific entropy increases by 0.007 kJ/kg.K. The power input t o t he electric heater is 2.50 kW. There is no other work or heat interaction between the system and the surroundings. Assuming an ambient temperature of 300 K, the irreversibility rate of the system is ____ kW (round off to two decimal places)
-
- i = 2.1 kW
- i = 3.1 kW
- i = 1.1 kW
- i = 0.1 kW
Correct Option: A
Irreversibility of system
= T0 .(∆ S)syst = 300[0.007]
I = 2.1 kJ/kg