-
Nitrogen gas (molecular weight 28) is enclosed in a cylinder by a piston, at the initial condition of 2 bar, 298 K and 1 m3. In a particular process, the gas slowly expands under isothermal condition, until the volume becomes 2 m3. Heat exchange occurs with the atmosphere at 298 K during this process.
The work interaction for the nitrogen gas is
-
- 200 kJ
- 138.6 kJ
- 2 kJ
- –200 kJ
- 200 kJ
Correct Option: B
| Work interaction , | 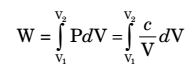 |
| W = cln |  |  | ||
| V1 |
| W = P1V1ln |  |  | ||
| V1 |
W = 2 × 105 × ln(2)
W = 2 × 105 × 0.693
W = 1.386 × 105 J = 138.6 kJ

