Thermodynamics Miscellaneous
- A Carnot cycle is having an efficiency of 0.75. If the temperature of the high temperature reservoir is 727°C. What is the temperature of low temperature reservoir?
-
View Hint View Answer Discuss in Forum
1 - T2 = 0.75 T1 T2 = 0.25 273 - 727
T2 = 0.25K
T2 = 727 – 250
[T2 – 23°C]Correct Option: B
1 - T2 = 0.75 T1 T2 = 0.25 273 - 727
T2 = 0.25K
T2 = 727 – 250
[T2 – 23°C]
- The slopes of constant volume and constant pressure lines in the T-s diagram are _______ and _________ respectively.
-
View Hint View Answer Discuss in Forum
Tds = du + pdv
Constant volume
Tds = dv (dv = 0)
Tds = CVdt
dt = CV 
.......(i) ds T
Tds = du – vdp
Constant pressure
dt = CP 
....(ii) ds T Correct Option: B
Tds = du + pdv
Constant volume
Tds = dv (dv = 0)
Tds = CVdt
dt = CV 
.......(i) ds T
Tds = du – vdp
Constant pressure
dt = CP 
....(ii) ds T
- A 1500 W electrical heater is used to heat 20 kg of water (cp = 4186 J/kgK) in an insulated bucket, from a temperature of 30°C to 80°C. If the heater temperature is only infinitesimally larger than the water temperature during the process, the change in entropy for the heater is _________ J/K and for water J/K.
-
View Hint View Answer Discuss in Forum
ds = mCIn Th Ti (20)(4186) In 353 303
dswater = 12786.99 J/kg
dsrod = – 12786.99 J/kgCorrect Option: D
ds = mCIn Th Ti (20)(4186) In 353 303
dswater = 12786.99 J/kg
dsrod = – 12786.99 J/kg
- If a closed system is undergoing an irreversible process, the entropy of the system
-
View Hint View Answer Discuss in Forum
(ds)uni ≥ 0
Correct Option: A
(ds)uni ≥ 0
- One kilogram of water at room temperature is brought into contact with a high temperature thermal reservoir. The entropy change of the universe is
-
View Hint View Answer Discuss in Forum
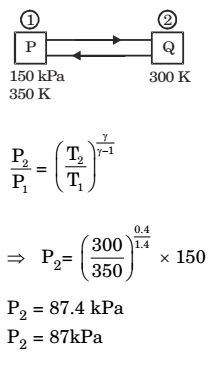
Correct Option: D


