Thermodynamics Miscellaneous
- A calorically perfect gas (specific heat at constant pressure 1000 J/kgK) enters and leaves a gas turbine with the same velocity. The emperature of the gas at turbine entry and exit are 1100 K and 400 K, respectively. The power produced is 4.6 MW and heat escapes at the rate of 300 kJ/s through the turbine casing. The mass flow rate of the gas (in kg/s) through the turbine is
-
View Hint View Answer Discuss in Forum
m 
h1 + C²1 gz1 
+ Q = m 
h2 + C²2 gz2 
+ w 2 2
m (h1 – h2) + Q = w
m (700) (1000) = (4600 + 300) ×103 J/s = 7kg/sCorrect Option: B
m 
h1 + C²1 gz1 
+ Q = m 
h2 + C²2 gz2 
+ w 2 2
m (h1 – h2) + Q = w
m (700) (1000) = (4600 + 300) ×103 J/s = 7kg/s
- An engine operates on the reversible cycles as shown in the figure. The work output from the engine (in kJ/cycle) is _______(correct to two decimals)
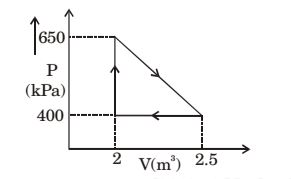
-
View Hint View Answer Discuss in Forum
Work output = ∫pdv
W = 1 × (2 - 5 - 2)(650 - 900) 2 W = 1 × (0.5)(250) 2
W = 62.5 kJ/cycleCorrect Option: C
Work output = ∫pdv
W = 1 × (2 - 5 - 2)(650 - 900) 2 W = 1 × (0.5)(250) 2
W = 62.5 kJ/cycle
- Which among the following relations is/are valid only for reversible process undergone by a pure substance?
-
View Hint View Answer Discuss in Forum
δQ = PdV + dU
Correct Option: D
δQ = PdV + dU
- One kilomole of an ideal gas is throttled from an initial pressure of 0.5 MPa to 0.1 MPa. The initial temperature is 300 K. The entropy change of the universe is
-
View Hint View Answer Discuss in Forum
Throttling, Ti = Tf
Henceds = RIn Pi Pf = 8.314In 0.5 0.1
8.314 ln 5
(ds) = 13.388 kJ/KCorrect Option: A
Throttling, Ti = Tf
Henceds = RIn Pi Pf = 8.314In 0.5 0.1
8.314 ln 5
(ds) = 13.388 kJ/K
- An ai r st andar d Ot t o cycl e has t her mal efficiency of 0.5 and the mean effective pressure of the cycle is 1000 kPa. For air, assume specific heat ratio γ = 1.4 and specific gas constant R = 0.287 kJ/kg.K. If the pressure and temperature at the beginning of the compression stroke are 100 kPa and 300 K, respectively, then the specific net work output of the cycle is ___ kJ/ kg
-
View Hint View Answer Discuss in Forum
η = 0.5 = 1 - 1 (r)γ-1 ∵ r = = compression ratio = VT = 1 + Vs VC VC
∵ Before compression,Total volume, VT = m.RT [let m = 1 kg] P VT = RT = 0.287 × 300 P 100
VT = 0.861 m³/kg
From equation (1)
⇒ r = 5.656⇒ VT = 5.656 VC VC = ⇒ 0.861 = 0.152m ³/kg 5.656
Swept volume, VS (r – 1).VC
= 4.656 × 0.152
= 0.70877 m³ /kg
Specific net work output of cycle,
W = Pmep × VS
= 1000 × 0.70877
Wnet =708.77 kJ/kgCorrect Option: C

η = 0.5 = 1 - 1 (r)γ-1 ∵ r = = compression ratio = VT = 1 + Vs VC VC
∵ Before compression,Total volume, VT = m.RT [let m = 1 kg] P VT = RT = 0.287 × 300 P 100
VT = 0.861 m³/kg
From equation (1)
⇒ r = 5.656⇒ VT = 5.656 VC VC = ⇒ 0.861 = 0.152m ³/kg 5.656
Swept volume, VS (r – 1).VC
= 4.656 × 0.152
= 0.70877 m³ /kg
Specific net work output of cycle,
W = Pmep × VS
= 1000 × 0.70877
Wnet =708.77 kJ/kg

