Direction: In a steam power plant operating on the Rankine cycle, steam enters the turbine at 4 MPa, 350°C and exists at a pressure of 15 kPa. Then it enters the condenser and exists as saturated water. Next, a pump feeds back the water to the boiler. The adiabatic efficiency of the turbine is 90%. The thermodynamic states of water and steam are given in the table. 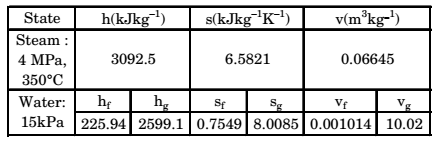
h is specific enthalpy, s is specific entropy and v the specific volume; subscript f and g denote saturated liquid state and saturated vapour state.
-
The net work output (kJ kg–1) of the cycle is
-
- 498
- 775
- 860
- 957
Correct Option: A
Given: Rankine cycle on T-S diagram
P3 = 4 MPa
T3 = 350°C
ηt = 0.9
h3 =3092.5 kJ/kg
s3 = s4 = 6.5821 kJ/kgk
h1 = 225.94 kJ/kg = hf
hg = 2599.1 kJ/kg 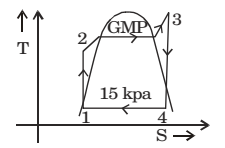
hfg = hg – hf = 2373.16 kJ/kg
s1 = sf = 0.7549 kJ/kgK
sg = 8.0085 kJ/kgK
sfg = 8.0085 – 0.7949 = 7.2536 kJ/kg K
Also, s3 = s4 = 6.5821
∴ s4 = s1 + x sfg
= 0.7549 + x (7.2536) = 6.5821
⇒ x = 0.8
h4 = h1 + xhfg
= 225.94 + (0.8 × 2375.16) = 2132.4 kJ/kg
Net work output of the cycle
= ηt (h3 – h4)
= 0.9 (3092.5 – 2132.4)
= 864 kJ/kg
≈ 860 kJ/kg
Heat supplied to the cycle = h3 – h2
Since we do not have information regarding h2, therefore neglect compressor work and take
h2 = h1
∴ Qs = 3092.5 – 2866 kJ/kg = 2863 kJ/kg

